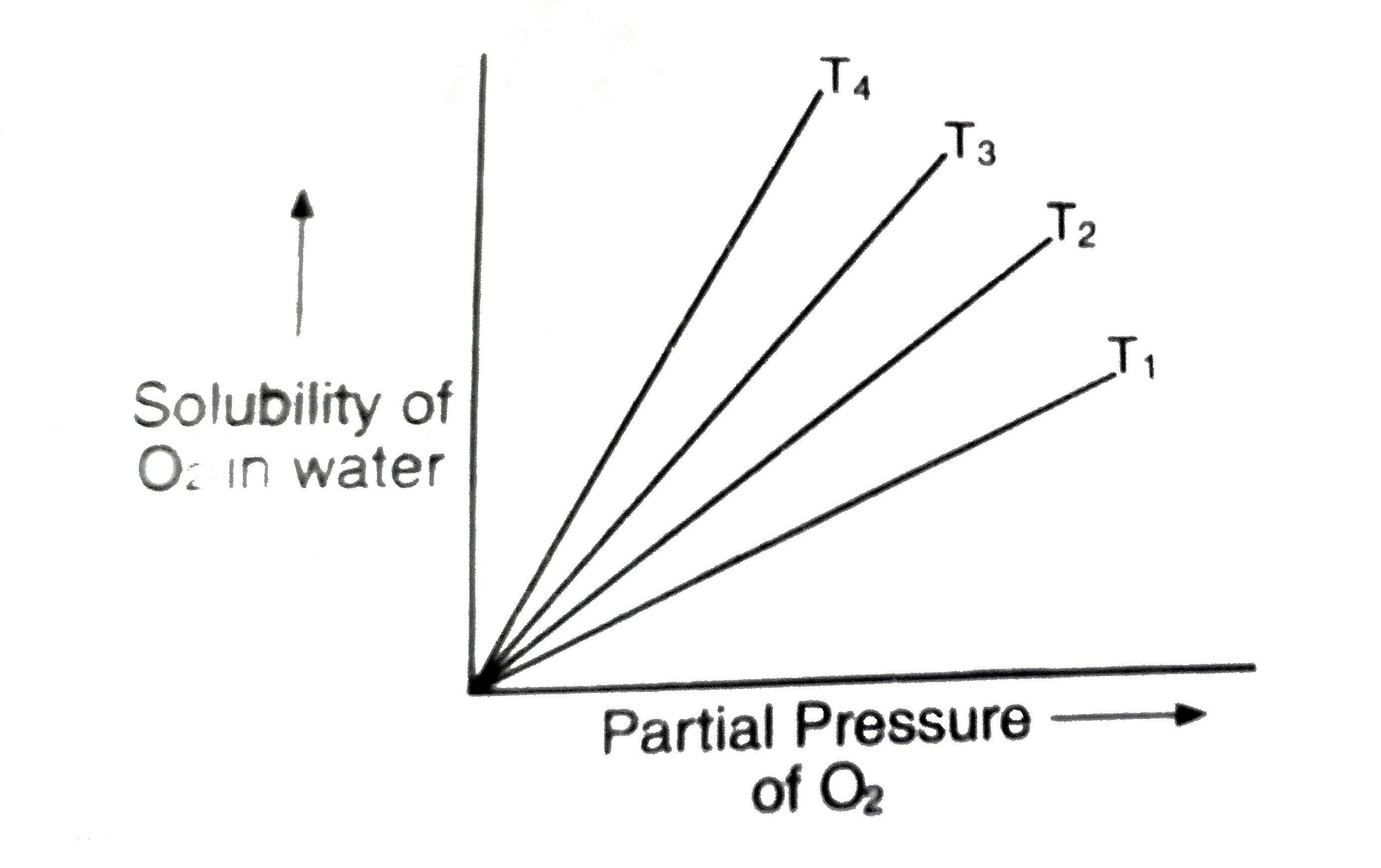
For a given gas, a graph is shown between compressibility factor (Z) and Pressure (P).Select the incorrect statement(s) about the various temperature relations.a)Temperature T1 must be above critical temperature (TC).b)Temperature T2 may

By A Mystery Man Writer

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
Compressibility factor (Z) is plotted against pressure at different te
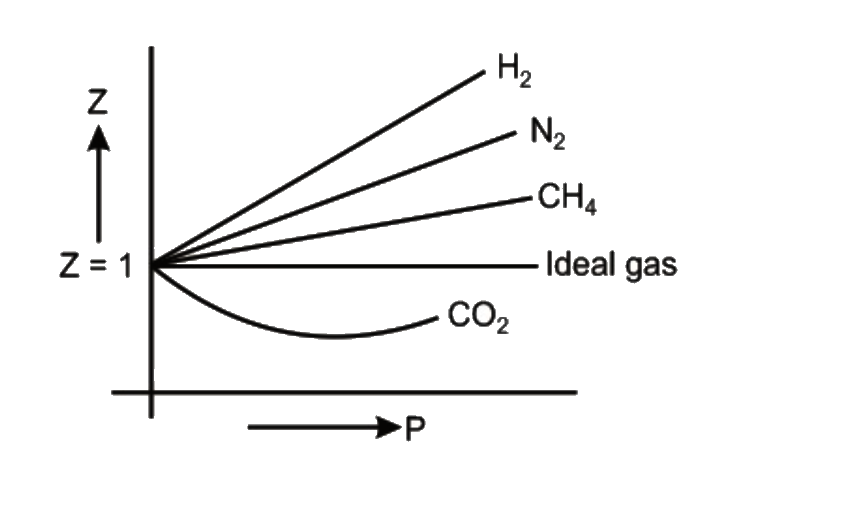
Consider the graph between compressibility factor Z and pressure P

The following graph is plotted between compressibility factor Z versus pressure of a gas at different temperatures.Which of the following statements is /are correct?

The graph of compressibility factor (Z) :vs: P one mole of a real gas is shown in following diagram. The graph is plotted constant temperature 273 K. If the slope of graph

compressibility Factor v/s Pressure/ Temperature Graph . States of Matter Class XI.

compressibility Factor v/s Pressure/ Temperature Graph . States of Matter Class XI.

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with Compressibility Factor

Compressibility Factor Z Important Concepts and Tips for JEE Main
- Compressibility factor Z = PV / nRT is plotted against pressure as
- Oil & Gas Softwares on X: Gas Compressibility Factor Calculator

- For a given gas, a graph is shown between compressibility factor

- physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

- Two extensions of the compressibility factor Z correlation (sub
- TOP 10 BEST Sprinkler Installation in Los Angeles, CA - Updated 2024 - Yelp

- Gymshark, Intimates & Sleepwear

- Feliz hermosa mujer joven en ropa de invierno de moda con un sombrero de punto, chaqueta, bufanda y jeans se encuentra cerca de una moto con árboles de navidad y regalos en

- MC Miller MS Girls/Ladies Leggings - MC Miller Middle School

- Shop Healthy Keto Peanut Butter Cups

