Compressibility factor Z = PV / nRT is plotted against pressure as

By A Mystery Man Writer
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2
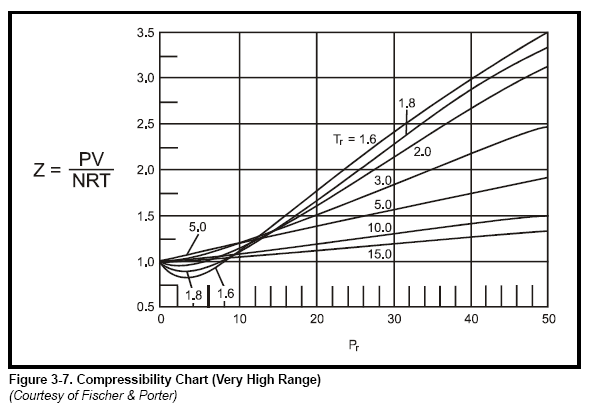
Chapter 3 - Physical Properties of Fluids: Gas Compressibility Factor

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Thermodynamics Exam 1 (Ch.1-3) Defs. Flashcards

COMPRESSIBILITY FACTOR

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Real Gases vs Ideal Gases & the Compressibility Factor

Solved Problem 2. ( 30 points) The ideal gas law can

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced

What is compressibility factor? What is its value for ideal gas

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

The given graph represents the variation of Z (compressibility factor) vs. P three real gases A, B and C. Identify the correct statementFor the gas A, a=0 and its dependence on P
- 3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

- The role of the compressibility factor Z in describing the

- Air Compressibility Factor Table - EnggCyclopedia
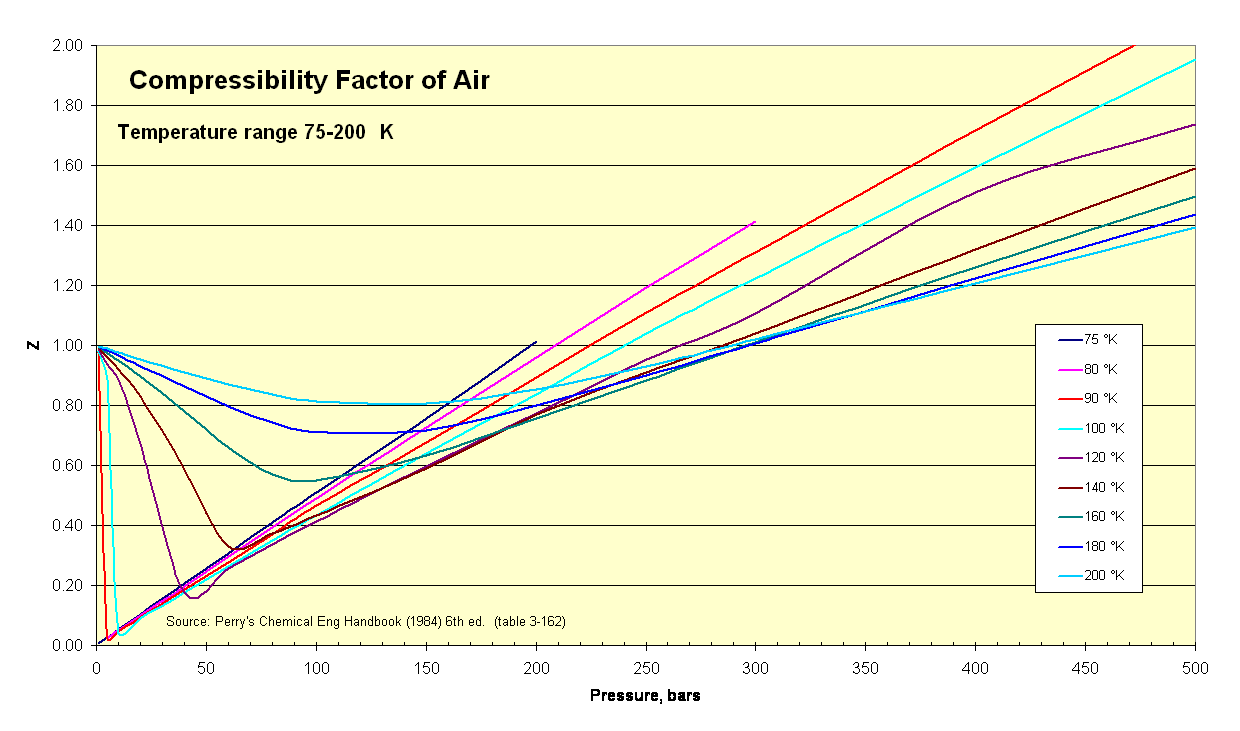
- physical chemistry - Is the compressibility factor smaller or

- Gas Z Factor Calculator: Dranchuk-Abou-Kassem · PVT Solver

- George ladies ultimate opaque tights 1pk - george ladies ultimate

- Men's Fanatics Branded Navy/Red Atlanta Braves Chip In Pullover Hoodie

- Powerful Yoga Poses can Actually Tone and Lift Up Your Saggy

- ZARA A PERFUME IN ROSE 3.4 oz (100 ml) EDP Spray NEW & SEALED - Helia Beer Co

- women's maidenform t-shirt bra red w/ white dots underwire size 32D MSRP $34
