physical chemistry - Is the compressibility factor smaller or

By A Mystery Man Writer
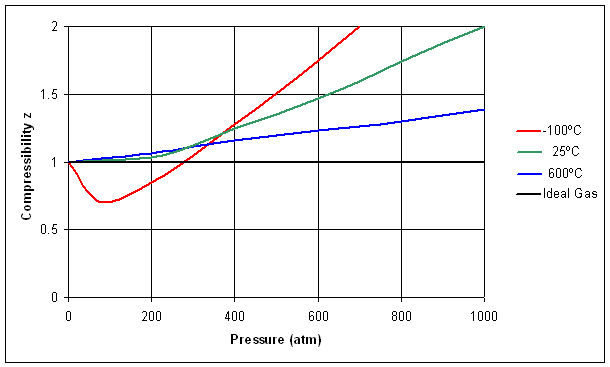
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

Compressibility factor - Wikipedia

gas laws - Graph of compressibility factor vs pressure when real

Compressibility factor (gases) - Knowino

Deviation Of Real Gas From Ideal Gas Behavior
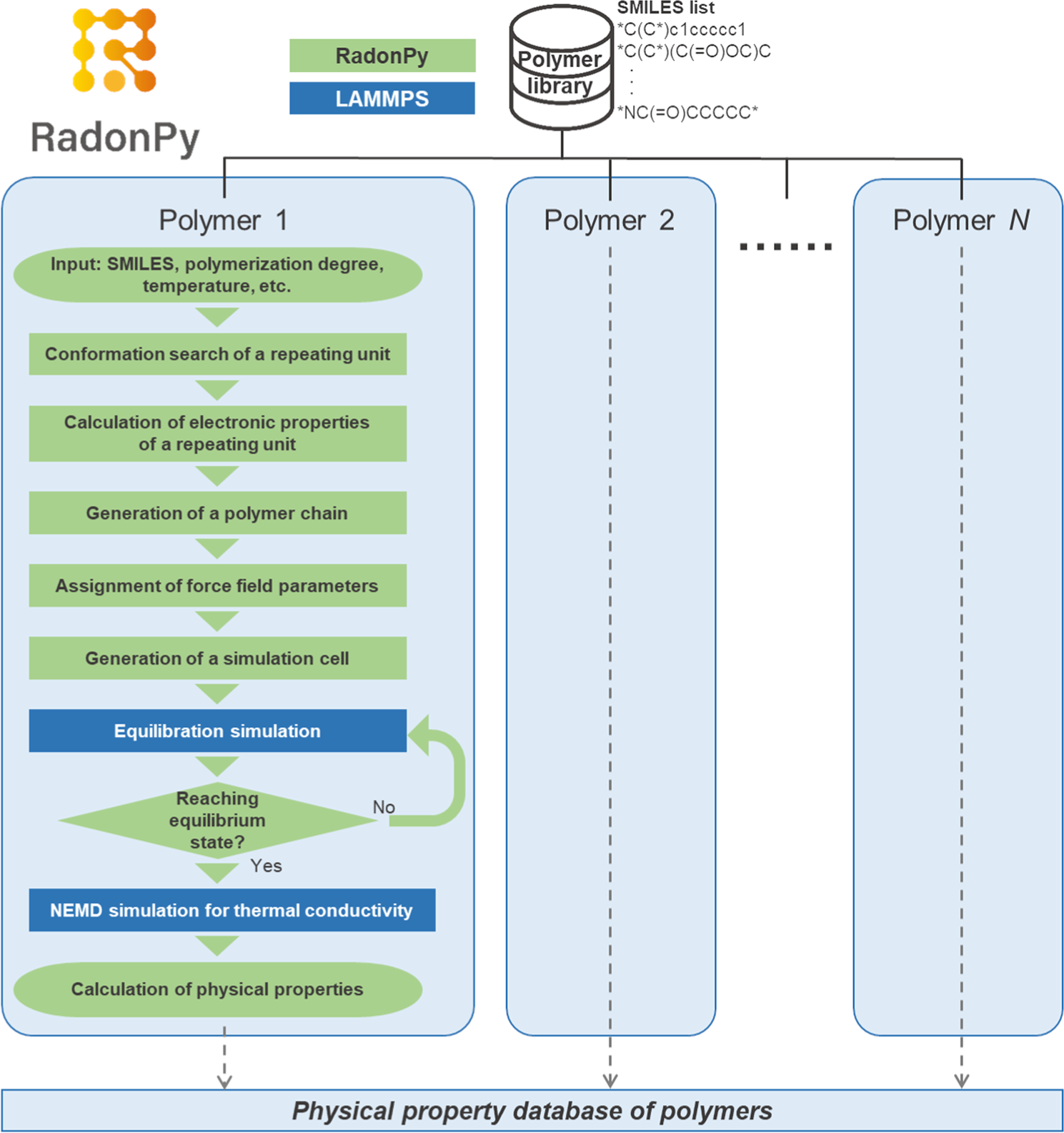
RadonPy: automated physical property calculation using all-atom classical molecular dynamics simulations for polymer informatics
Gas Laws – First Year General Chemistry

Physical Chemistry The Compression Factor (Z) [w/1 example

physical chemistry - Compressibility Factor Graph - Which gas attains a deeper minimum? - Chemistry Stack Exchange

Non-Ideal Gas Behavior Chemistry: Atoms First
- Compressibility factor (Z) for a van der Waals real gas at critical point is

- Gas compressibility factor Z: Ideal gas vs Real gas
- Answered: (a)Using the compressibility chart,…

- The role of the compressibility factor Z in describing the volumetric behavior of gases

- Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora
- Black Maxi Dress - Off-the-Shoulder Dress - Mermaid Maxi Dress - Lulus

- Madonna convida um time de astros e estrelas da música pop para festejar em 'Bitch, I'm Madonna

- Buy EROS White Shapewear Tanktop, Horizontal Striped, U-Neck, Slim Fit, Sleeveless Underwear for Men 2024 Online
:format(webp)/https://static-sg.zacdn.com/p/eros-8395-3793743-2.jpg)
- Goddess Audrey Soft Cup Bra, Nude Wireless Goddess Soft cup Bras – Bras & Honey USA

- Yes, my husband knows I'm here. Classic Thong

