Quantum Numbers for Atoms - Chemistry LibreTexts
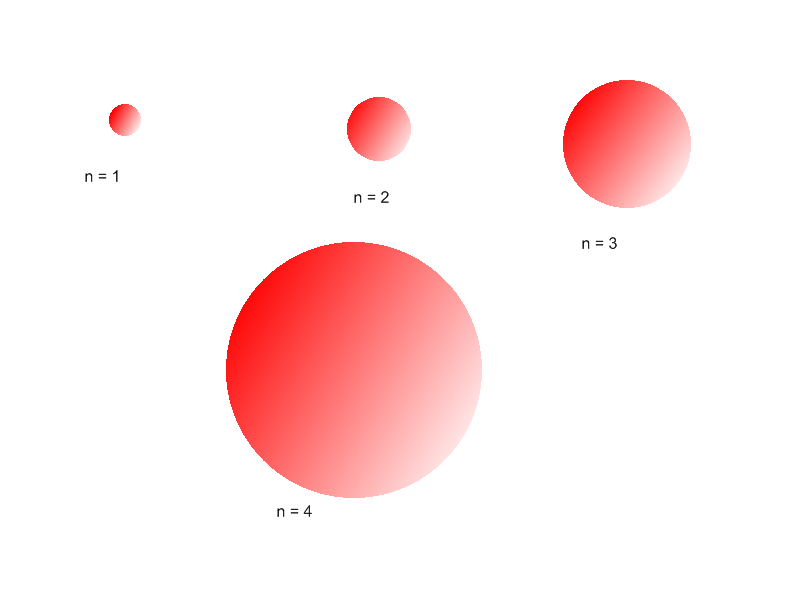
By A Mystery Man Writer
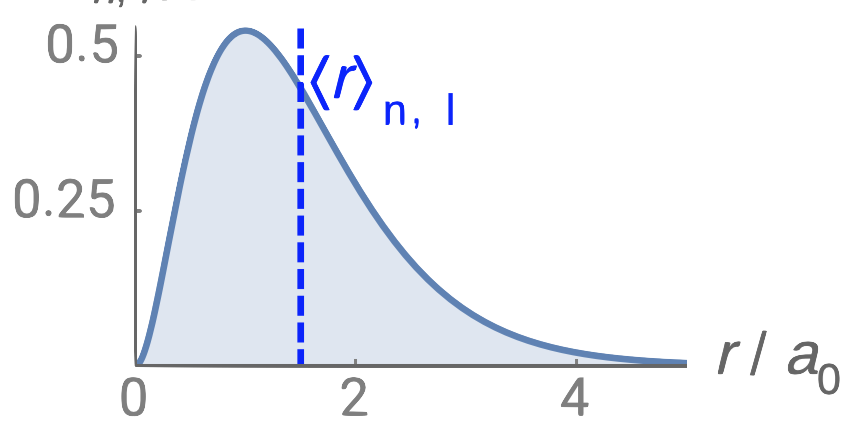
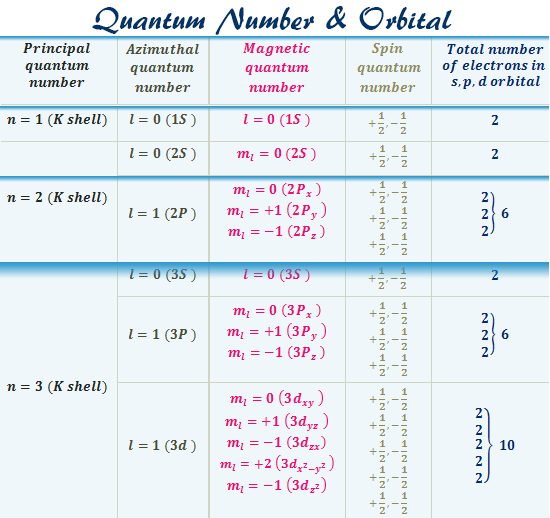
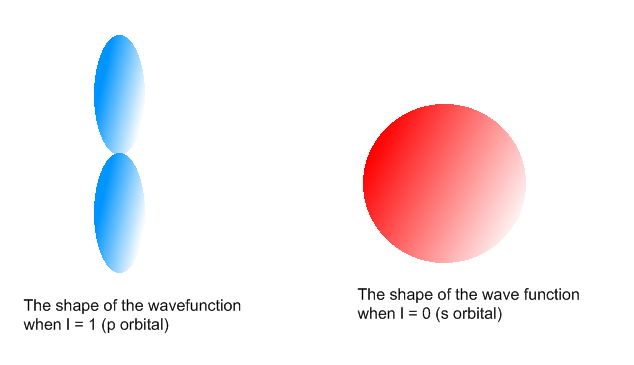
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The combination of all quantum numbers of all electrons in an atom is …
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The combination of all quantum numbers of all electrons in an atom is described by a wave function that complies with the Schrödinger equation. Each electron in an atom has a unique set of quantum numbers; according to the Pauli Exclusion Principle, no two electrons can share the same combination of four quantum numbers.

2.2: The Four Quantum Numbers - Chemistry LibreTexts

Chemical bond - Wikipedia

Impressions: Robinson's Brutus Awards For 2015, Part, 42% OFF
2.2.2: Quantum Numbers and Atomic Wave Functions - Chemistry

GeneralChemistry1 Q2 Module-1 Quantum Mechanical Descriptions v5-1.pdf - Senior High School NOT General Chemistry 1 Quarter 2 - Module 1 Quantum

Quantum Numbers for Atoms - Chemistry LibreTexts

Las shs gen.chem-melc_1_q2_week-1

Science Activity Sheet: Quarter 2 - MELC 1 Week 1, PDF, Atomic Orbital
3.5 Quantum Mechanics and The Atom – Chemistry LibreTexts
Quantum Numbers For Atoms Chemistry LibreTexts, 43% OFF
Quantum Numbers for Atoms - Chemistry LibreTexts

Quantum Numbers For Atoms Chemistry LibreTexts, 43% OFF
- Wallace Table Lamp Antique Brass - Splendor Home : Target
- Vintage Brass Bunny, Sleeping Bunny, Rabbit, Shelf / Mantel Decor

- MARZXIN 2024 Tankini Swimsuit for Women Floral Two Piece Bathing

- Miss Perfect Shapewear Damen - Bauchweg Unterhose Damen (S-3XL) Body Shaper seamless Miederhose Bauch weg - nahtlos & formend

- Deyllo Women's Embroidered Lace Unlined Bra 1/2 Cup Demi Sheer See Through Underwire Bras Non Padded,Milk coffee 34C