Solved Show that the compressibility factor of van der Waals

By A Mystery Man Writer
Answer to Solved Show that the compressibility factor of van der Waals
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

thermodynamics - Negative Pressures in Van der Waals Equation of State - Chemistry Stack Exchange
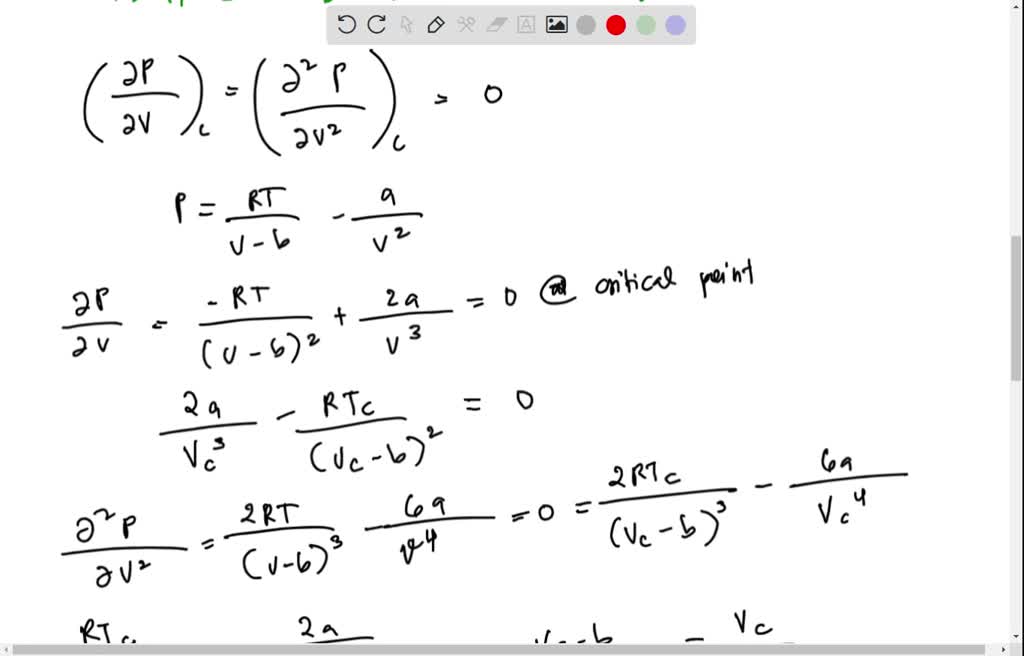
SOLVED: 1) Estimate/ Calculate the critical constants (pc, Vc, and Tc) for a gas molecule whose van der Waals parameters are a = 1.32 atm dm^6 mol^-2 and b = 0.0436 dm^3

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at

At high pressure, the compressibility factor for one mole of van der w

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Solved 2. (20 points) At low pressures, the compressibility
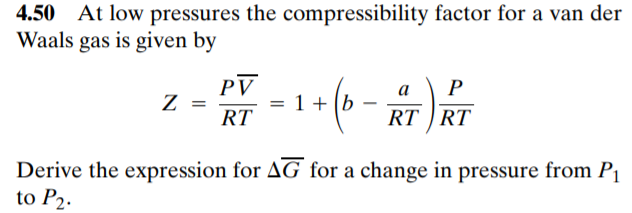
Solved 4.50 At low pressures the compressibility factor for

SOLVED: The fugacity of a van der Waals gas can be determined using the expression for the compressibility factor Z. The expression for fugacity of a van der Waals gas is given

Solved We showed, for a van der Waals gas, that the

A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

Van Der Waals Equation of State - an overview
- Compressibility Factor of Carbon Dioxide - Maple Application Center

- Compressor and jet vacuum system:, by Maryambotshekan

- Figure 3 from A Simple Equation Of State For Calculating The Compressibility Factor Of Pure Fluids Based On The Virial EOS

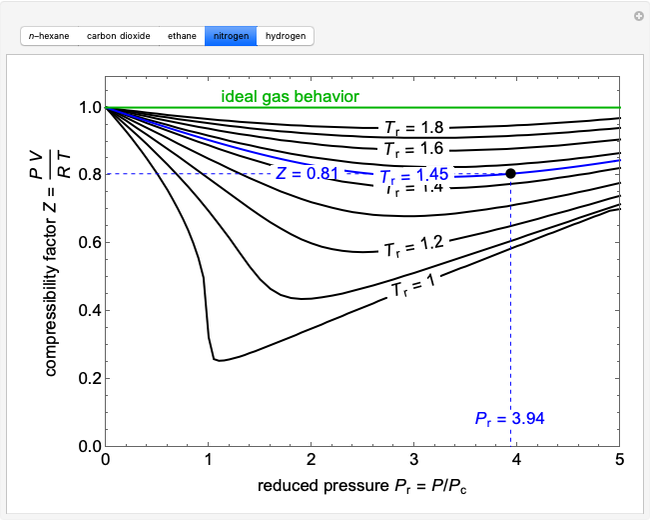
- Compressibility Factor Charts - Wolfram Demonstrations Project
- Solved RT B 2. The compressiblity factor for a gas is